FDA
The firings included 20 individuals in the FDA's Office of Neurological and Physical Medicine Devices.
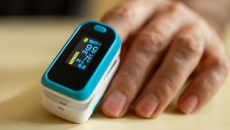
The new draft guidance aims to help improve the accuracy of pulse oximeters in patients across the range of skin pigmentations.
This week's top stories include baby tech company Owlet pulling its connected-sock wearables following an FDA warning letter, and TeamHealth's victory in a lawsuit accusing UnitedHealthcare of engaging in unfair reimbursement practices.
Dr. Amy Abernethy, principal deputy commissioner at the U.S. FDA, discusses the FDA's role in balancing innovation, regulation and safety during the coronavirus pandemic.
The new algorithms integrate with Eko's digital stethoscope.
Both companies are ramping up the pace of their FDA clearances.